- English
- فارسی
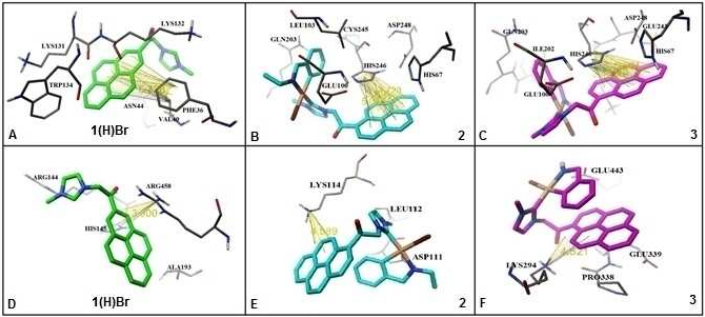
Luminescent Palladacycles Containing a Pyrene Chromophor; Synthesis, Biological and Computational Studies of the Interaction with DNA and BSA
Kazem Karami, Azar Ramezanpour, Mostafa Zakariazadeh, Azar Shahpiri, Mahshid Kharaziha, Akram Kazeminasab
One of the most active areas within the field of bioorganometallic chemistry, complexes of N‐heterocyclic carbenes (NHCs), have recently gained interest. Herein, we report two luminescent palladium N‐heterocyclic carbene complexes; namely [Pd{(C,N)‐C6H4CH2NH(CH2CH3)}(1)] (2) and [Pd{(C,N)‐C6H4CH2NH2}(1)] (3) (1=1‐methyl‐3‐(2‐oxo‐2‐(pyren‐1‐yl)ethyl)‐2,3‐dihydroimidazol‐2‐ylidene) which were synthesized from the reaction of luminescent imidazolium salt (1(H)Br) and binuclear Palladacycles. The interactions of them with CT‐DNA evaluated via absorption, emission and CD spectral techniques as well as measurements of viscosity and thermal denaturation and the results have been shown that they bounded to CT‐DNA by intercalation and groove binding modes. The in vitro cytotoxicity of compounds 2–3 and 1(H)Br on human breast (MCF‐7) and cervical epithelial carcinoma (HeLa) cancer cells lines, indicated the wide range of anticancer activities of them with low IC50 values. Moreover, based on the protein binding ability studies, the intrinsic fluorescence of BSA could be strongly quenched by compounds via a static quenching mechanism. Competitive binding study using Eosin, Digoxin and Ibuprofen as site markers, indicated that the compounds could bind to sites I and II on BSA structure. Finally, all data obtained from biophysical studies were validated by molecular modeling study. Computational results showed that palladium complexes have the potential for detection of mismatch DNA.